CPD frameworks
These frameworks, associated with all articles, prompt drafting of personal learning, reflection and planning.
Save your reflective note into your device or cloud
Fillable PDF frameworks
Adobe reader needed for tablets
Word frameworks - for reflective practice
Click to download
Reflection on a journal article
Stages of reflection on a situation
Reflection of a team, practice or group
External reference on reflection
Reflective practice in health care and how to reflect effectively
Koshy K, Limb C et al. International Journal of Surgical Oncology. 2017 2:e20
Leo M, Betts T. Prompt diagnosis of AF lowers risk of complications. Practitioner 2016;260(1797)11-17
Prompt diagnosis of AF lowers risk of complications
24 Oct 2016
AUTHORS
Dr Milena Leo MD, Clinical Research Fellow in Cardiac Electrophysiology
Dr Tim Betts MD FRCP, Consultant Cardiologist and Electrophysiologist
Oxford Heart Centre, John Radcliffe Hospital, Oxford, UK
Article
Abstract
Atrial fibrillation (AF) is the most common sustained heart rhythm disturbance. Estimates suggest an AF prevalence as high as 2% in adults with an exponential relationship with increasing age. AF is associated with a 1.5-2 fold increased risk of death, and is responsible for 20-30% of all strokes. There are strong relationships with hypertension, heart failure, coronary artery disease (CAD), valvular heart disease, obesity, diabetes mellitus, COPD, obstructive sleep apnoea, chronic kidney disease and lifestyle factors such as increased alcohol intake, strenuous physical exercise and smoking. Assessment should include physical examination (blood pressure measurement, cardiovascular examination to look for valvular heart disease or heart failure and lung examination looking for signs of lung disease or pulmonary oedema), blood tests, including urea and electrolytes, liver function tests, full blood count, blood glucose and thyroid function tests. Signs of haemodynamic instability or severe symptoms (unstable angina, evolving TIA or stroke, heart failure or severe bradycardia) should be promptly identified and lead to urgent referral to specialist care. The CHA2DS2-VASc risk stratification score is recommended to assess stroke risk in patients with AF. Oral anticoagulation should be offered to those with a CHA2DS2-VASc score ≥ 2, and considered for men with a score of 1 and women with a score of 2. Risk of severe bleeding with warfarin should also be assessed using the HAS-BLED score.
Atrial fibrillation (AF) is the most common sustained heart rhythm disturbance. It results from chaotic electrical activation of the top chambers of the heart, with a consequent loss of normal atrial contractility and an irregular and often fast ventricular rate.
The presentation of AF ranges from an unexpected finding in an individual with complete absence of symptoms (in up to one in three cases), through non-specific symptoms of lethargy and fatigue, to obvious and distressing palpitations and haemodynamic compromise. The lack of an organised atrial contraction during AF can also result in significantly reduced blood flow in the left atrium and activation of clotting factors, which in turn may lead to formation of a thrombus which can subsequently become dislodged. Patients with AF have a five-fold increased risk of ischaemic stroke, transient ischaemic attack or systemic embolism.
Estimates suggest an AF prevalence of 2% in adults with an exponential relationship with increasing age.1 Although a large minority of AF sufferers may have no obvious associated comorbidities, there is a strong relationship with hypertension, heart failure, coronary artery disease (CAD), valvular heart disease, obesity, diabetes mellitus, chronic obstructive pulmonary disease, obstructive sleep apnoea, chronic kidney disease and lifestyle factors such as increased alcohol intake, strenuous physical exercise and smoking.2
AF is associated with a 1.5-2 fold increased risk of death,3,4 and is responsible for 20-30% of all strokes.5,6
A recent meta-analysis has also documented an increased risk of cardiovascular disease and renal disease in patients with AF.7 White matter lesions in the brain, cognitive impairment, reduced quality of life, and depressed mood are also common in AF patients. Between 10 and 40% of AF patients are hospitalised each year.8
A new version of the European Society of Cardiology (ESC) guidelines has just been published to meet the growing demand for effective care of patients with AF.9 Following on from the clinical guideline CG180,10 NICE has also recently published a quality standard (QS93)11 where six main high priority areas for AF management are identified in order to reduce morbidity, mortality and acute admissions while improving quality of life, see table 1.
Diagnosis
As emphasised in the recent ESC guidelines, early diagnosis of AF is the only way to prevent complications such as stroke. GPs have a fundamental role in detecting AF and subsequently delivering appropriate care.
Opportunistic screening for silent AF is cost effective and therefore recommended in patients at risk, such as those 65 and over with hypertension, diabetes or a history of stroke. It can be performed using simple pulse palpation followed by ECG if an irregularity is detected. The Arrhythmia Alliance ‘Know Your Pulse’ campaign promotes routine pulse checks in GP surgeries and during flu clinics. Many new blood pressure monitors e.g. WatchBP Home12 can simultaneously detect pulse irregularity and display an AF icon.
In patients with intermittent palpitations suggestive of AF, prolonged ECG monitoring, ideally with 7-28 day event monitors, can be used to increase the likelihood of diagnosis. The type of monitor should be based on the frequency of symptoms e.g. a 24-hour monitor should only be used if symptoms occur every day. In patients with implanted defibrillators or pacemakers with an atrial lead, the atrial rhythm can be monitored continuously with an early diagnosis of atrial high rate episodes and/or AF.
New technologies, such as smartphone cases with ECG electrodes and smart watches, are now available. Despite not being formally evaluated against an established arrhythmia detection method, they seem to represent a practical and cost-effective way for correlation of symptoms with ECG and early AF detection.
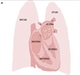
A NICE Medtech innovation briefing has recently become available on the AliveCor Kardia technology for detection of AF to provide objective information on this new diagnostic tool.13 Recorders are relatively inexpensive and allow an engaged patient to take ownership of the investigation and management of their condition, see figure 1.
Management
Once the diagnosis of AF has been made, a comprehensive assessment and initial management of the patient should be undertaken at the point of first medical contact. The patient should be given advice on lifestyle and risk factor management and key information to facilitate shared decision making. Referral to secondary care should be undertaken if treatment fails to control symptoms or ventricular rate, or if more specialised management is needed, for example in the case of difficult decisions regarding the balance of risks and benefits of anticoagulation. GPs and specialists should work together as part of a multidisciplinary AF team.
Assessment should include physical examination (blood pressure measurement, cardiovascular examination for valvular heart disease or heart failure and lung examination for signs of lung disease or pulmonary oedema), blood tests, including urea and electrolytes, liver function tests, full blood count, blood glucose and thyroid function tests.
The following five aspects should be considered:
Haemodynamic instability or severe symptoms
Signs of haemodynamic instability or severe symptoms (unstable angina, evolving TIA or stroke, heart failure or severe bradycardia) should be promptly identified and lead to urgent referral of the patient to specialist care.
Precipitating factors
Precipitating factors (e.g. thyrotoxicosis, sepsis, pneumonia or postoperative AF) and underlying cardiovascular conditions should be identified. Transthoracic echocardiography is often used to investigate underlying causes and guide management. It is recommended if a rhythm control strategy is contemplated, when there is suspicion of underlying heart disease (heart murmur, abnormal ECG or symptoms), to guide anticoagulation risk stratification or
in patient groups where AF is less common, e.g. in the younger patient.
It may not be required in asymptomatic individuals in whom anticoagulation is mandated for other reasons.
As understanding of the patho-physiology of AF and the number of known aetiologies has increased together with improvements in the ability to image and diagnose heart disease, any underlying concealed or manifest heart disease can be identified in most cases of AF, with progressive reduction in the number of cases that could be defined as lone AF. For this reason, the new ESC guidelines recommend avoiding using this term.
Stroke and bleeding risks
The patient’s stroke and bleeding risks and their need for anticoagulation should be defined. The CHA2DS2-VASc score, see table 2, is recommended to assess stroke risk.
Oral anticoagulation (OAC) should be offered to patients with a CHA2DS2-VASc score ≥ 2, and considered for men with a score of 1 and women with a score of 2, balancing the expected stroke reduction, bleeding risk, and patient preference.
Importantly, age > 65 conveys a relatively high and continuously increasing stroke risk that also potentiates other risk factors (such as heart failure and male gender), while female gender does not appear to increase stroke risk in the absence of other stroke risk factors.
The risk of severe bleeding with warfarin should be assessed using the HAS-BLED score, see table 3.
A score ≥ 3 indicates that caution is required when starting anticoagulant therapy, however a high bleeding risk score or a perceived high risk of bleeding (cognitive dysfunction, frequent falls, frailty) should generally not result in withholding OAC as a net benefit from OAC often persists. Ideally, bleeding risk factors should be identified and treatable factors corrected.
OAC should be used in all patients with increased stroke risk as it can prevent up to two-thirds of ischaemic strokes in AF patients and can reduce mortality by a quarter.14,15
The need for regular monitoring and dose adjustments with warfarin, and the potential for multiple food and drug interactions, results in suboptimal time in the therapeutic range and a high rate of non-compliance and treatment cessation. A range of non-vitamin K oral anticoagulants (NOACs)16-19 have been approved for use in non-valvular AF, see table 4. Edoxaban is the most recent NOAC to be approved by NICE.19
The NOACs do not require regular monitoring as their anticoagulant effect is predictable. As their excretion is affected by renal function, dose adjustment is required in cases of renal failure. The only NOAC with an available antidote is dabigatran, although antidotes to the other NOACs are under development. The lack of antidote has not been shown to be detrimental and a number of studies have shown that following severe bleeds patients on NOACS have better outcomes than those on warfarin.20
Deciding between warfarin and the different NOACs is a complex process that should take into account not only the patient’s age and comorbidities, but also NICE guidance based on trial entry criteria, patient preference and compliance. The new 2016 ESC guidelines recommend NOACs as the first-line anticoagulant in eligible patients because they prevent strokes as effectively or slightly better than warfarin and are associated with less intracranial bleeding and fewer deaths. Warfarin still remains a valid treatment for stroke prevention in AF and should be the first choice in patients ineligible for NOACs, such as those with mechanical heart valves, or who are intolerant.
Aspirin is not an option for thromboembolic stroke prevention in AF. It is not as effective as anticoagulants at preventing stroke. In the very old it has a similar rate of bleeding as warfarin. In patients with high stroke risk and contraindications to anticoagulation due to high risk of bleeding.
NHS England is currently evaluating percutaneous left atrial appendage occlusion.21 This approach has recently been demonstrated in randomised controlled trials and large registries to be as effective as warfarin at lowering the risk of stroke but does not increase bleeding risk.
Rate control
The heart rate in AF should be promptly evaluated and a rate control strategy should be adopted in the first instance.
Rate control is an integral part of AF management to prevent tachycardia cardiomyopathy in persistent AF and it may be sufficient to improve AF-related symptoms. It should be adopted as a first-line strategy, aiming for a target resting heart rate of < 100 bpm.
Standard beta blockers e.g. bisoprolol, or the calcium antagonists verapamil or diltiazem are equally effective as initial monotherapy, while digoxin is useful only in combination with other drugs. Calcium antagonists should be avoided in patients with left ventricular dysfunction.
Catheter ablation of the atrioventricular node to create complete heart block combined with insertion of a permanent pacemaker is a final resource in cases of poor ventricular control in the elderly.
Rhythm control
Symptoms should be reassessed after the achievement of rate control to evaluate the need for rhythm control.
The Modified EHRA symptoms score, see table 5, is now recommended to describe symptom severity. A score of 2b or higher might identify patients benefiting from a rhythm control strategy.
A rhythm control strategy should be strongly considered when symptoms persist despite good rate control, especially for newly diagnosed AF and in the case of a reversible cause. Rhythm control strives to restore and maintain sinus rhythm, preserving atrial contractility as well as a normal heart rate.
The options for rhythm control include electrical cardioversion if the AF is persistent, plus antiarrhythmic drugs and catheter ablation. External or internal electrical cardioversion can acutely restore sinus rhythm in 90-95% of patients.
If AF has been continuous for 48 hours or more, a minimum of three weeks of therapeutic anticoagulation is required before cardioversion can be safely performed. Unfortunately, only 20-30% of patients are still in sinus rhythm one year after cardioversion.
This percentage can be increased by catheter ablation and/or addition of a long-term antiarrhythmic drug.
Antiarrhythmic drugs for long-term rhythm control include standard beta blockers (e.g. bisoprolol), flecainide, propafenone, amiodarone, sotalol or dronedarone), see table 6. Flecainide can also be used as a ‘pill in the pocket strategy’ (a single 200-300 mg dose taken at the onset of the attack) in patients with infrequent, long-lasting episodes of AF, and no left ventricular dysfunction or valvular or ischaemic heart disease. Unfortunately, most drugs merely reduce the frequency of AF rather than eliminate it entirely and are commonly accompanied by limiting side effects.
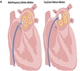
Catheter ablation is an invasive rhythm control strategy performed through femoral vein needle punctures.
It aims to isolate the pulmonary veins from the left atrium electrically, see figure 2. The veins are often the source of ectopics that act as electrical triggers, initiating and perpetuating AF. Catheter ablation is indicated in highly symptomatic patients with paroxysmal or persistent AF in whom drug therapy has failed and a rhythm control strategy is preferred. Current techniques have a 90% success rate for paroxysmal AF and a 70-80% success rate for persistent AF but this is often only achieved after multiple procedures.22,23 There is a small but real risk of serious complications, such as tamponade, stroke, vascular complications and death, even in the hands of experienced clinicians.
Conclusion
AF is a very common condition associated with an increased risk of morbidity and mortality. Prompt diagnosis and management is fundamental to prevent complications, particularly stroke, and alleviate symptoms.
Opportunistic screening for silent AF is recommended in at-risk patients. In addition to prolonged ECG monitoring, new technologies such as smartphone cases with ECG electrodes and smart watches can increase the likelihood of diagnosis in patients with paroxysmal palpitations.
Rate control and anticoagulation, with rhythm control in symptomatically limited patients, are cornerstones of AF management. A variety of anticoagulants are now available for stroke prevention. Catheter ablation and/or medications are feasible options for rhythm control.
The patient should have a central role in their care process and be given appropriate information to facilitate shared decision making. Care should be integrated, with close and continuous interaction between GPs and specialists working as a multidisciplinary team.
REFERENCES
1 Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213-20
2 Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N
3 Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113(5):359-64
4 Benjamin EJ, Wolf PA, D'Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98(10):946-52
5 Cotter PE, Martin PJ, Ring L et al. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013;80(17):1546-50
6 Sanna T, Diener HC, Passman RS et al. CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370(26):2478-86
7 Odutayo A, Wong CX, Hsiao AJ et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482
8 Steinberg BA, Kim S, Fonarow GC et al. Drivers of hospitalization for patients with atrial fibrillation: Results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J 2014; 167(5):735-42
9 Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016 Aug 27 [Epub ahead of print]
10 National Institute for Health and Care Excellence. CG180. Atrial fibrillation: the management of atrial fibrillation. NICE. London. 2014
11 National Institute for Health and Care Excellence. QS93. Atrial fibrillation. NICE. London. 2015
12 National Institute for Health and Care Excellence MTG13. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension. NICE. London. 2014
13 National Institute for Health and Care Excellence. MIB35. AliveCor Heart Monitor and AliveECG app for detecting atrial fibrillation. NICE. London. 2015
14 Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857-67
15 Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62
16 National Institute for Health and Care Excellence. TA249. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation. NICE. London. 2012
17 National Institute for Health and Care Excellence. TA256. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. NICE. London. 2012
18 National Institute for Health and Care Excellence. TA275. Apixaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. NICE. London. 2013
19 National Institute for Health and Care Excellence. TA355. Edoxaban for preventing stroke and systemic embolism in people with non-valvular atrial fibrillation. NICE. London. 2015
20 Skaistis J, Tagami T. Risk of fatal bleeding in episodes of major bleeding with new oral anticoagulants and vitamin K antagonists: A systematic review and meta-analysis. PLoS One 2015 Sep 18;10 (9):e 0137444
21 National Institute for Health and Care Excellence. IPG349. Percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation for the prevention of thromboembolism. NICE. London. 2014
22 Ganesan AN, Shipp NJ, Brooks AG et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2(2):e004549
23 Oral H, Scharf C, Chugh A et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus leftatrial ablation. Circulation 2003; 108(19):2355-60
24 National Institute for Health and Care Excellence. IPG563. Percutaneous endoscopic laser balloon pulmonary vein isolation for atrial fibrillation. NICE. London. 2016
ARTICLE IN PDF
EXTERNAL WEBLINKS
Atrial Fibrillation Association
NICE
A decision aid for patients with atrial fibrillation is available from the website
http://guidance.nice.org.uk/CG180/PatientDecisionAid/pdf/English
Arrhythmia Alliance
Smartphone camera study
Brasier N, Raichle CJ, Dorr M et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019; 21(1):41-47
ESC guidelines 2016
Kirchof P, Beussi S, Kotecha D et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-2962

 = Registered users
= Registered users = Paid-up subscribers
= Paid-up subscribers