CPD frameworks
These frameworks, associated with all articles, prompt drafting of personal learning, reflection and planning.
Save your reflective note into your device or cloud
Fillable PDF frameworks
Adobe reader needed for tablets
Word frameworks - for reflective practice
Click to download
Reflection on a journal article
Stages of reflection on a situation
Reflection of a team, practice or group
External reference on reflection
Reflective practice in health care and how to reflect effectively
Koshy K, Limb C et al. International Journal of Surgical Oncology. 2017 2:e20
Javanmard-Emamghissi HM, Humes DJ. Diagnosing and managing colorectal cancer. Practitioner July/Augt 2018;262(1817):17-21
Diagnosing and managing colorectal cancer
25 Jul 2018
AUTHORS
Miss Hannah M Javanmard-Emamghissi BMedSci BMBS MRCS, Surgical Registrar
Mr David J Humes BSc MBBS PhD FRCS, Associate Professor of Surgical Epidemiology, Honorary Consultant Colorectal Surgeon
Nottingham Digestive Disease Centre and Biomedical Research Unit, Nottingham University Hospital NHS Trust, Nottingham, UK
Article
Abstract
Colorectal cancer is the fourth most common cancer in the UK. Around 42,000 new colorectal cancers are diagnosed every year, and it is the second most common cause of cancer deaths in the UK. Most cancers are thought to develop from colonic adenomas and incidence is strongly related to age.The majority of cancers are left sided and typically present with a change in bowel habit, blood in the stool or colicky abdominal pain. Rectal cancers can present with fresh red bleeding and large tumours can cause tenesmus (the intense and frequent desire to defecate, with little or no stool passed). Right-sided cancers most often present with anaemia. As the diameter of the caecum is large and the bowel contents at this stage liquid, it is uncommon for right-sided cancers to present with obstructive symptoms. In both right- and left-sided cancers occasionally the patient may notice an abdominal mass or inexplicable weight loss. Lifestyle risk factors include high consumption of red or processed meat, low fibre intake, sedentary lifestyle, obesity, smoking and high alcohol consumption. Patients with IBD are also at increased risk and all colitis patients should be offered surveillance colonoscopy starting ten years after onset of symptoms. Colonoscopy is the gold standard investigation for suspected colorectal cancer. Surgery is the only potentially curative treatment for colorectal adenocarcinoma and is offered to around two-thirds of patients.
Colorectal cancer is the fourth most common cancer in the UK population. Approximately 42,000 new colorectal cancers are diagnosed every year, and it is the second most common cause of cancer death in the UK.1 The incidence of colorectal cancer increases with age and it is diagnosed most often in the seventh and eighth decades.1 It is thought to develop from colonic adenomas.
The prompt detection of colorectal cancer is vital as there is good evidence that survival is significantly improved by early diagnosis and intervention.2,3
Presentation
Colorectal cancer presents differently according to its location. More than 50% of cancers are located in the rectum and left colon.1 Right-sided cancers most often present with anaemia. As the diameter of the caecum is large and the bowel contents at this stage liquid, it is uncommon for right-sided cancers to present with obstructive symptoms.4 Cancers arising from the descending colon typically present with a change in bowel habit, blood in the stool or colicky abdominal pain. Rectal cancers can present with fresh red bleeding however, large tumours can cause tenesmus (the intense and frequent desire to defecate, with little or no stool passed).
In both right- and left-sided cancers occasionally the patient may notice an abdominal mass or weight loss.
Referral
The NICE guideline on referral of patients with suspected cancer (NG12) recommends that the following patients should be referred for an urgent appointment with a specialist within two weeks via the suspected cancer pathway referral route:5
• Aged 40 and over with unexplained weight loss and abdominal pain
• Aged 50 and over with unexplained rectal bleeding
• Aged under 50 with rectal bleeding and unexplained abdominal pain or change in bowel habit or weight loss or iron deficiency anaemia
• Aged 60 and over with iron-deficiency anaemia or changes in bowel habit
• Any patient with a positive faecal occult blood test (FOBT)
• Any patient with a rectal or abdominal mass
Risk factors for colorectal cancer
Genetic risk factors
There are two well characterised genetic conditions Lynch syndrome (formerly known as hereditary non-polyposis colorectal cancer) and familial adenomatous polyposis (FAP).6
Lynch syndrome is an autosomal dominant condition that accounts for 3% of colorectal cancers.6 It presents with early colorectal cancer typically diagnosed in the fifth decade and is associated with a number of other cancers including endometrial, stomach, small bowel, urothelial (renal pelvis, ureter, bladder) and ovarian cancer.6
Recent NICE guidance (DG27) recommends that all patients diagnosed with colorectal cancer should have immunohistochemical testing of their cancer for evidence of Lynch syndrome.7 If positive the patient and their family can be offered genetic counselling and screening. The Amsterdam II criteria (see box 1) can aid in identification of patients at risk of Lynch syndrome based on family history alone who can subsequently be referred for genetic testing.
FAP is inherited in an autosomal dominant fashion due to a mutation in the adenomatous polyposis coli (APC) tumour suppressor gene on chromosome 5q although 20% of cases are caused by new mutations. FAP accounts for < 1% of colorectal cancers.8
The condition presents with hundreds of adenomatous polyps of the colon in the second and third decades of life.
Those affected also develop duodenal adenomas along with well characterised extra-colonic manifestations of the disease.
Dietary and lifestyle risk factors
There is strong evidence that high consumption of red meat (beef, pork and lamb) and processed meat increases the risk of developing colorectal cancers.9 There is also an increased risk in those with a high fat diet. In contrast diets rich in wholegrains, dietary fibre and dairy products are considered to be protective.9
Being physically active is associated with a reduction in the risk of colorectal cancer, whereas being overweight or obese is associated with an increased risk.9 A large meta-analysis found the risk to be increased by 5% for every 5 kg/m2 the patient is above normal BMI.7 Smoking is associated with an increased risk of developing colorectal cancer7 as is high alcohol consumption (more than 30 g per day).9
Predisposing conditions
Inflammatory bowel disease (Crohn’s disease and ulcerative colitis) is associated with an increase in the risk of developing colorectal cancer. The risk to these patients remains the same as the general population in the first few years following their diagnosis but rises to 2% at ten years.10 All colitis patients should be offered surveillance colonoscopy ten years after the onset of symptoms.10,11 The frequency of subsequent surveillance is determined by initial surveillance colonoscopy findings.10,11
Confirming diagnosis
Endoscopy
Colonoscopy is the gold standard investigation for suspected colorectal cancer.12 It visualises the entire large bowel, is very sensitive for colorectal cancer and allows for both tissue diagnoses and polypectomy.13
A complete colonoscopy, whereby the caecum is intubated, is reliant on adequate bowel preparation, patient tolerance and technical skill.14 Colonoscopy is an invasive test which carries a risk of bleeding, perforation and complications as a consequence of sedative medication such as myocardial infarction and pneumonia.14
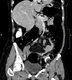
CT colonography
CT colonography or virtual colonoscopy (see figure 1) is non-invasive, widely available and has replaced the use of barium enemas in most UK centres.4,15
It is used as an alternative to endoscopy in patients who cannot tolerate bowel preparation e.g. the frail and elderly, as it is able to detect polypoid lesions as small as 6 mm.4
Staging
Once a diagnosis of cancer has been made the patient will undergo staging with a CT of the chest, abdomen and pelvis with intravenous contrast.12 This allows for assessment of local invasion, lymph node spread and distant metastasis (most commonly in the liver and lungs). For rectal cancers, the patient will also have an MRI of the pelvis to determine the necessity for preoperative radiotherapy or chemotherapy in the hope of reducing local recurrence rates.12,15
A baseline blood test for the colorectal tumour marker carcinoembryonic antigen (CEA) is also taken.
All cases of colorectal cancer should be discussed at a specialist colorectal multidisciplinary team (MDT) meeting to determine the optimum treatment plan.
Treatment
Surgery
Surgery is the only potentially curative treatment for colorectal adenocarcinoma. Around two-thirds of patients diagnosed with bowel cancer are offered surgical management.3 The aim of surgery is to optimise the oncological outcome while maintaining as normal bowel function as possible.
The exact procedure performed depends on the location of the tumour and depth of invasion (see table 1).
Increasingly laparoscopic surgery is being used, with more than half of major resections in 2014-2015 completed laparoscopically.3 NICE recommends laparoscopic surgery in patients who are felt to be suitable for either laparoscopic or open procedures.12
Stomas
A defunctioning (or loop) stoma is formed from a bowel segment upstream from the area of anastomosis, which diverts stool to protect the patient from bowel content leakage while the anastomosis heals. These types of stomas are frequently seen following rectal surgery as the rectum has a poor blood supply and heals more slowly compared with the rest of the colon. These stomas do not have to be permanent and discussions can be had with the surgeon at a later stage to consider restoration of bowel continuity.
A permanent end colostomy or ileostomy may be formed as part of surgical treatment.
Surgical management of metastases
Patients with liver and lung metastases can be considered for resection, in situ ablation or oncological treatments.15
If the liver disease is considered resectable it can be performed as a single- or two-stage procedure combined with the bowel resection depending on the patient’s fitness and specialist MDT input.
Chemotherapy/radiotherapy
Patients with Dukes B and C disease may be offered adjuvant chemotherapy to reduce risk of recurrence (see table 2).12,15 There is evidence of improved survival rates even in Dukes D patients treated with chemotherapy.12,15
Rectal cancers can be downsized or the risk of local recurrence reduced with preoperative chemotherapy or radiotherapy.15
Decisions on appropriate treatment should be made at MDT meetings in conjunction with discussions with the patient.
Palliative management
Palliative management may involve best supportive care only, palliative chemotherapy/radiotherapy or palliative stenting in obstructing tumours. Palliative stenting cannot be performed in rectal cancers as it causes intractable tensesmus.12
Emergency management
In patients presenting as an emergency with obstruction treatment options include: emergency surgery, colonic stenting as a bridge to elective resection or as a palliative procedure and a defunctioning stoma as a palliative procedure.
Outcomes
The overall five-year survival rate for patients diagnosed with colorectal cancer is 59%.1 The survival rates can be further stratified according to histological features. There are several commonly used staging systems including Dukes staging and TNM (see table 2).
A large Europe-wide population-based study found the average five-year survival rate for colon cancer in the European Union to be 57% for both women and men.17 The best survival rate was in Germany, with 62.2% of patients surviving five years post diagnosis.17
In comparison the same study found that the five-year survival rate in the UK was 51.8%.17 The five-year survival rates when broken down by region show better survival rates in Northern Ireland (55%) and the worst survival rates in Wales (49.9%).17 No conclusive evidence to account for this difference has yet been presented. Five-year relative survival rates of patients diagnosed with colorectal cancer based on stage at diagnosis are shown in table 2.2
Screening
In England, Northern Ireland and Wales patients are offered screening by FOBT every two years from the age of 60 to 75. In Scotland this is now offered from the age of 50. There is strong evidence that screening in this way significantly reduces mortality from colorectal cancer by 15-33% as demonstrated in three large randomised controlled trials.18,19,20
There is a move towards abandoning FOBT in favour of faecal immunochemical testing. This has already happened in Scotland, and is based on evidence of improved detection sensitivity and better patient uptake of the single sample test compared with the FOBT which involves multiple samples.21,22
In addition to faecal testing, the Bowel Scope programme which offers a single flexible sigmoidoscopy to all men and women aged 55 in England is being rolled out. This has recently been shown to confer 30% improvement in mortality over a 17-year follow-up period.23
Monitoring and follow-up
All patients who undergo resection for colorectal cancer should have formal specialist follow-up. There is evidence that the main benefit of follow-up is earlier detection of metastatic disease.15 There is no conclusive evidence as to what is the optimal follow-up regimen.15 Most notably the FACS randomised clinical trial looked at intensive six monthly CEA, CT and combined CEA/CT follow-up and concluded that compared with minimal follow-up (a single CT scan at 12-18 months) there was earlier detection of recurrence and a higher rate of surgical treatment with curative intent, but overall no survival benefit.24
NICE recommends six monthly CEA measurement for the first three years and a minimum of two CT scans in the same period.12 Patients are also offered colonoscopy at one and five years to aid detection of further adenomatous polyps.12 The exact follow-up regimen may vary according to local guidance. Patients should be reinvestigated at any point if there is clinical, radiological or biochemical evidence of disease recurrence.12
Late effects
The late effects of colorectal cancer and its subsequent treatment include radiation proctitis, change in bowel and bladder control, sexual dysfunction and anterior resection syndrome.25
The management of these problems requires a specialist approach and involvement of the MDT.
Competing interests: None
REFERENCES
1 Cancer Research UK. Bowel cancer statistics www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer [Last accessed July 3 2018]
2 National Cancer Intelligence Network. Colorectal cancer survival by stage. www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage [Last accessed July 3 2018]
3 Braun M, Hill J, Kuryba A et al. National Bowel Cancer Audit. 2014 [Last accessed July 3 2018] www.hqip.org.uk
4 Steele R. Colorectal surgery: A companion to specialist surgical practice. Chapter 3. In Clark S. Ed. 6th edition. Elsevier. London. 2018. p27
5 The National Institue for Health and Care Excellence. NG12. Suspected cancer: recognition and referral. NICE. London. 2015 www.nice.org.uk/guidance/ng12
[Last accessed July 3 2018]
6 Clark S, Latchford A. Colorectal surgery: A companion to specialist surgical practice. Chapter 4. In Clark S. Ed. 6th edition. Elsevier. London. 2018. p37
7 National Institute for Health and Care Excellence. DG27. Molecular testing strategies for Lynch syndrome in people with colorectal cancer. NICE. London. 2017 www.nice.org.uk/guidance/dg27
8 Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138;2044-58
9 World Cancer Research Fund/American Institue of Cancer Research. Diet, nutrition, physical activity and colorectal cancer. 2018 www.wcrf.org/dietandcancer/colorectal-cancer [Last accessed July 5 2018]
10 Eaden J, Mayberry J. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 2002;51(Suppl 5):v10-2
11 Cairns SR, Scholefield JH, Steele RJ et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59(5):666-89
12 National Institute for Health and Care Excellence. CG131. Colorectal cancer: diagnosis and management. NICE. London. 2011 [Last accessed July 12 2018]
13 Rex DK, Rahmani EY, Haseman JH et al. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology 1997;112(1):17-23
14 Bowles CJA, Leicester R, Romaya C et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004;53(2):277 LP-283
15 Scottish Intercollegiate Guidelines Network. SIGN 126. Diagnosis and management of colorectal cancer. SIGN. Edinburgh. 2011 www.sign.ac.uk/assets/sign126.pdf [Last accessed July 3 2018]
16 McDermott FD, Heeney A, Kelly ME et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102(5):462-79
17 De Angelis R, Sant M, Coleman MP et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol 2014;15(1):23-34 [Last accessed July 5 2018]
18 Kronborg O, Fenger C, Olsen J et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348(9040):1467-71
19 Hardcastle JD, Chamberlain JO, Robinson MH et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348(9040):1472-77
20 Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999;91(5):434-37
21 Hol L, van Leerdam ME, van Ballegooijen M et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010;59(01):62 LP-68
22 Guittet L, Bouvier V, Mariotte N et al. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut 2007;56(2):210 LP-214
23 Atkin W, Wooldrage K, Parkin DM et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK flexible sigmoidoscopy screening randomised controlled trial. Lancet 2017;389(10076):1299-1311
24 Primrose JN, Perera R, Gray A et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer. JAMA 2014;311(3):263 [Last accessed July 5 2018]
25 Macmillan Cancer Support. Managing the long-term consequences of colorectal and anal cancer: Guidance for health care professionals 2015 [Last accessed July 3 2018]
ARTICLE IN PDF
PRESET SEARCHES
US National Cancer Institute
PDQ® (Physician Data Query) is NCI's cancer database of peer-reviewed, regularly updated evidence-based, referenced summaries
Colorectal screening
Colon
NICE- evidence search
Colorectal cancer
PubMed
PubMed is a database of the US National Library of Medicine/National Institutes of Health

 = Registered users
= Registered users = Paid-up subscribers
= Paid-up subscribers